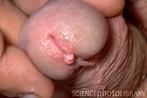
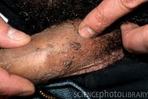
Warts can be transmitted thru sex
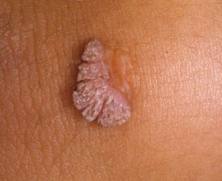
And you thought warts on your hands were bad !
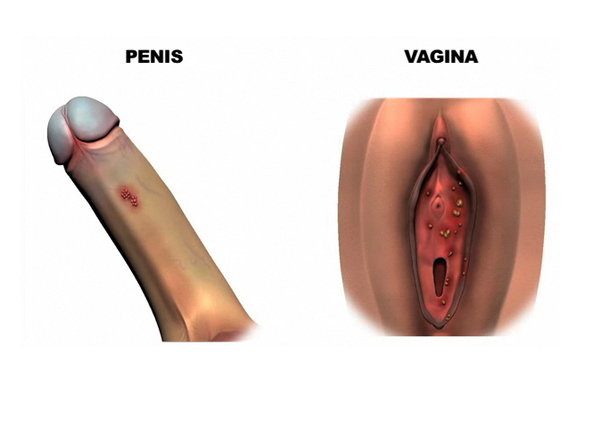
Even worse, the wart virus, sometimes know as Human Papilloma Wart Virus, or HPV, can occur on the genitals.
It can cause abnormal pap smears and cancer of the cervix in women.
And even cancer of the foreskin ( if you still have one ) in men.
Stats say that about one third of College age men and women have the HPV in their system, and that most get rid of it spontaneously.
But even better than getting rid of it is preventing it.
And this can be done by vaccinating you women ( and men ) with the HPV vaccine , which sometimes goes by the trade name Gardasil ). This can be done anytime, but the best time is at age 15, because
Even worse, the wart virus, sometimes know as Human Papilloma Wart Virus, or HPV, can occur on the genitals.
It can cause abnormal pap smears and cancer of the cervix in women.
And even cancer of the foreskin ( if you still have one ) in men.
Stats say that about one third of College age men and women have the HPV in their system, and that most get rid of it spontaneously.
But even better than getting rid of it is preventing it.
And this can be done by vaccinating you women ( and men ) with the HPV vaccine , which sometimes goes by the trade name Gardasil ). This can be done anytime, but the best time is at age 15, because
- 15 year olds are not likely to be sexually active yet
- 15 year olds respond to vaccines better than older people
Wart Vaccine for Boys
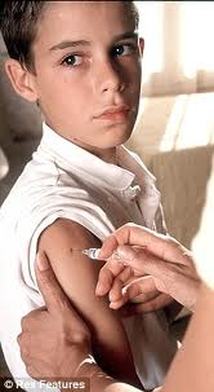
February 27, 2012
The American Academy of Pediatrics (AAP) has published new guidelines for the use of the human papillomavirus vaccine and, for the first time, has specifically recommended use of the vaccine in adolescent boys as well as girls.
The recommendations were published online February 27 and in the March print issue of Pediatrics.
The vaccine was recommended for girls in 2006, but even though at that time the AAP said the vaccine could be used in boys, it was not specifically recommended for that population.
The new recommendations were spurred in part by mounting evidence that the HPV vaccine is effective as prophylaxis against genital warts in both males and females. HPV infection has been associated with increased risk for cervical cancer, anal cancer, and oropharyngeal cancer.
The AAP recommends that the vaccine be administered at 11 to 12 years of age in both boys and girls. Their rationale is 2-fold: First, the vaccine is most effective if it is administered before the individual begins engaging in sexual activity, mainly because the vaccine is inactive against HPV strains acquired before vaccination. Second, children mount the most robust antibody responses to the vaccine when they are between the ages of 9 and 15 years, the AAP says.
Two HPV vaccines are currently available in the United States, but there are differences in their approved indications. Quadrivalent HPV vaccine (HPV4, Gardasil, Merck) is the only vaccine approved for use in boys. Bivalent HPV vaccine (HPV2, Cervarix, GlaxoSmithKline) is only approved for use in girls; HPV4 is also approved for girls.
The American Academy of Pediatrics (AAP) has published new guidelines for the use of the human papillomavirus vaccine and, for the first time, has specifically recommended use of the vaccine in adolescent boys as well as girls.
The recommendations were published online February 27 and in the March print issue of Pediatrics.
The vaccine was recommended for girls in 2006, but even though at that time the AAP said the vaccine could be used in boys, it was not specifically recommended for that population.
The new recommendations were spurred in part by mounting evidence that the HPV vaccine is effective as prophylaxis against genital warts in both males and females. HPV infection has been associated with increased risk for cervical cancer, anal cancer, and oropharyngeal cancer.
The AAP recommends that the vaccine be administered at 11 to 12 years of age in both boys and girls. Their rationale is 2-fold: First, the vaccine is most effective if it is administered before the individual begins engaging in sexual activity, mainly because the vaccine is inactive against HPV strains acquired before vaccination. Second, children mount the most robust antibody responses to the vaccine when they are between the ages of 9 and 15 years, the AAP says.
Two HPV vaccines are currently available in the United States, but there are differences in their approved indications. Quadrivalent HPV vaccine (HPV4, Gardasil, Merck) is the only vaccine approved for use in boys. Bivalent HPV vaccine (HPV2, Cervarix, GlaxoSmithKline) is only approved for use in girls; HPV4 is also approved for girls.
Vaccinate boys and girls against HPV

Among the AAP's updated recommendations are that:
The AAP recommends that because the HPV vaccine will not prevent infection from all types of HPV types, cervical screening should continue after HPV vaccination.
The organization also says that administration of the vaccine should not alter physicians' recommendations regarding use of barrier methods for preventing HPV and other sexually transmitted diseases.
The AAP urges that use of the vaccine be covered by all public and private health insurance.
More information on implementing the guidelines, including guidance on supply, payment, coding, and liability issues, is available on the AAP's Web site.
The AAP recommends that because the HPV vaccine will not prevent infection from all types of HPV types, cervical screening should continue after HPV vaccination.
The organization also says that administration of the vaccine should not alter physicians' recommendations regarding use of barrier methods for preventing HPV and other sexually transmitted diseases.
The AAP urges that use of the vaccine be covered by all public and private health insurance.
More information on implementing the guidelines, including guidance on supply, payment, coding, and liability issues, is available on the AAP's Web site.
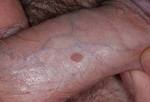
What does a wart on a penis look like ?
THE "OFFICIAL" OFFICIAL RECOMMENDATIONS
1. Girls 11 through 12 years of age
should be immunized routinely with 3 doses of HPV4 or HPV2, administered intramuscularly at 0, 1 to 2, and 6 months. The vaccines can be administered starting at 9 years of age at the discretion of the physician.
2. All girls and women 13 through 26 years of age who have not been immunized previously or have not completed the full vaccine series should complete the
series.
3. Boys 11 through 12 years of age should be immunized routinely with 3 doses of HPV4, administered intramuscularly at 0, 1 to 2, and 6 months. The vaccine can be given starting at 9 years of age at the discretion of the physician.
4. All boys and men 13 through 21 years of age who have not been
immunized previously or have not completed the full vaccine series should receive HPV4 vaccine.
5. Men 22 through 26 years of age who have not been immunized previously or have not completed the full vaccine series may receive HPV4 vaccine. Cost-efficacy models do not justify a stronger recommendation in this age group.
6. Special effort should be given to immunizing men who have sex with men up to 26 years of age who have not been immunized previously or have not completed the full vaccine series.
7. Previous sexual activity is not a contraindication to HPV immunization or completion of the immunization series. Patients infected with 1 HPV type may still benefit from protection against remaining HPV types in the vaccine. Testing for previous exposure to HPV is not recommended. HPV vaccine can be administered when a female patient has an
abnormal or equivocal Papanicolaou test result. There is no known therapeutic (as opposed to prophylactic) benefit from the HPV vaccines.
8. HIV-infected people of either gender, 9 through 26 years of age, who have not been immunized previously or have not completed the full vaccine series should receive or com plete their series with HPV4.
9. HPV vaccines can be administered at the same visit as all other recommended vaccines.
10. HPV vaccine can be administered in these special circumstances:
a. when a patient is immunocompromised because of disease or medication
b. when a female patient is breastfeeding
11. HPV vaccine is not recommended during pregnancy. The practitioner should inquire about pregnancy in sexually active female patients, but a pregnancy test is not required
before starting the immunization series. If a vaccine recipient becomes pregnant, subsequent doses should be postponed until completion of the pregnancy. It is recommended that women who become pregnant while receiving HPV vaccine be reported to registries that have been developed to record data on outcomes (HPV2: 1-888-452-9622; HPV4: 1-800-986-8999).
12. Because HPV vaccine will not prevent infection attributable to all high-risk HPV types, cervical cancer screening recommendations (ie, Papanicolaou testing) should
continue to be conducted in women who have received HPV vaccine.
13. Administration of HPV vaccine does not change current counseling recommendations for use of barrier methods for the prevention of HPV and other sexually transmitted infections as well as discussion about healthy choices about sexual activity, including condoms and abstinence.
14. HPV immunization of children 9 years of age and older should be covered by all public and private health insurers.
CONTRAINDICATIONS :
HPV4 should not be given to people with a history of immediate hypersensitivity to yeast, or to pregnant women.
PRECAUTIONS
Immunizations should be deferred for people with moderate or severe acute illness. Because syncope can occur in adolescents after injections and has been reported after HPV vaccine, vaccine recipients should sit or lie down for 15 minutes after administration.
IMPLEMENTATION
These updated recommendations for HPV immunization will have considerable operational and fiscal effect on pediatric practice. Therefore, the AAP has developed implementation guidance on supply, payment, coding, and liability issues; these documents can be found at www.aapredbook.org/ implementation
1. Girls 11 through 12 years of age
should be immunized routinely with 3 doses of HPV4 or HPV2, administered intramuscularly at 0, 1 to 2, and 6 months. The vaccines can be administered starting at 9 years of age at the discretion of the physician.
2. All girls and women 13 through 26 years of age who have not been immunized previously or have not completed the full vaccine series should complete the
series.
3. Boys 11 through 12 years of age should be immunized routinely with 3 doses of HPV4, administered intramuscularly at 0, 1 to 2, and 6 months. The vaccine can be given starting at 9 years of age at the discretion of the physician.
4. All boys and men 13 through 21 years of age who have not been
immunized previously or have not completed the full vaccine series should receive HPV4 vaccine.
5. Men 22 through 26 years of age who have not been immunized previously or have not completed the full vaccine series may receive HPV4 vaccine. Cost-efficacy models do not justify a stronger recommendation in this age group.
6. Special effort should be given to immunizing men who have sex with men up to 26 years of age who have not been immunized previously or have not completed the full vaccine series.
7. Previous sexual activity is not a contraindication to HPV immunization or completion of the immunization series. Patients infected with 1 HPV type may still benefit from protection against remaining HPV types in the vaccine. Testing for previous exposure to HPV is not recommended. HPV vaccine can be administered when a female patient has an
abnormal or equivocal Papanicolaou test result. There is no known therapeutic (as opposed to prophylactic) benefit from the HPV vaccines.
8. HIV-infected people of either gender, 9 through 26 years of age, who have not been immunized previously or have not completed the full vaccine series should receive or com plete their series with HPV4.
9. HPV vaccines can be administered at the same visit as all other recommended vaccines.
10. HPV vaccine can be administered in these special circumstances:
a. when a patient is immunocompromised because of disease or medication
b. when a female patient is breastfeeding
11. HPV vaccine is not recommended during pregnancy. The practitioner should inquire about pregnancy in sexually active female patients, but a pregnancy test is not required
before starting the immunization series. If a vaccine recipient becomes pregnant, subsequent doses should be postponed until completion of the pregnancy. It is recommended that women who become pregnant while receiving HPV vaccine be reported to registries that have been developed to record data on outcomes (HPV2: 1-888-452-9622; HPV4: 1-800-986-8999).
12. Because HPV vaccine will not prevent infection attributable to all high-risk HPV types, cervical cancer screening recommendations (ie, Papanicolaou testing) should
continue to be conducted in women who have received HPV vaccine.
13. Administration of HPV vaccine does not change current counseling recommendations for use of barrier methods for the prevention of HPV and other sexually transmitted infections as well as discussion about healthy choices about sexual activity, including condoms and abstinence.
14. HPV immunization of children 9 years of age and older should be covered by all public and private health insurers.
CONTRAINDICATIONS :
HPV4 should not be given to people with a history of immediate hypersensitivity to yeast, or to pregnant women.
PRECAUTIONS
Immunizations should be deferred for people with moderate or severe acute illness. Because syncope can occur in adolescents after injections and has been reported after HPV vaccine, vaccine recipients should sit or lie down for 15 minutes after administration.
IMPLEMENTATION
These updated recommendations for HPV immunization will have considerable operational and fiscal effect on pediatric practice. Therefore, the AAP has developed implementation guidance on supply, payment, coding, and liability issues; these documents can be found at www.aapredbook.org/ implementation